Alle weiteren Präsentationen unter https://boerse-social.com/austrianworldwideroadshow

Valneva - Advancing Vaccines for Better Lives (18.01.2022)

Valneva - Disclaimer (18.01.2022)

Valneva - Summary (18.01.2022)

Valneva - Research & Development (18.01.2022)
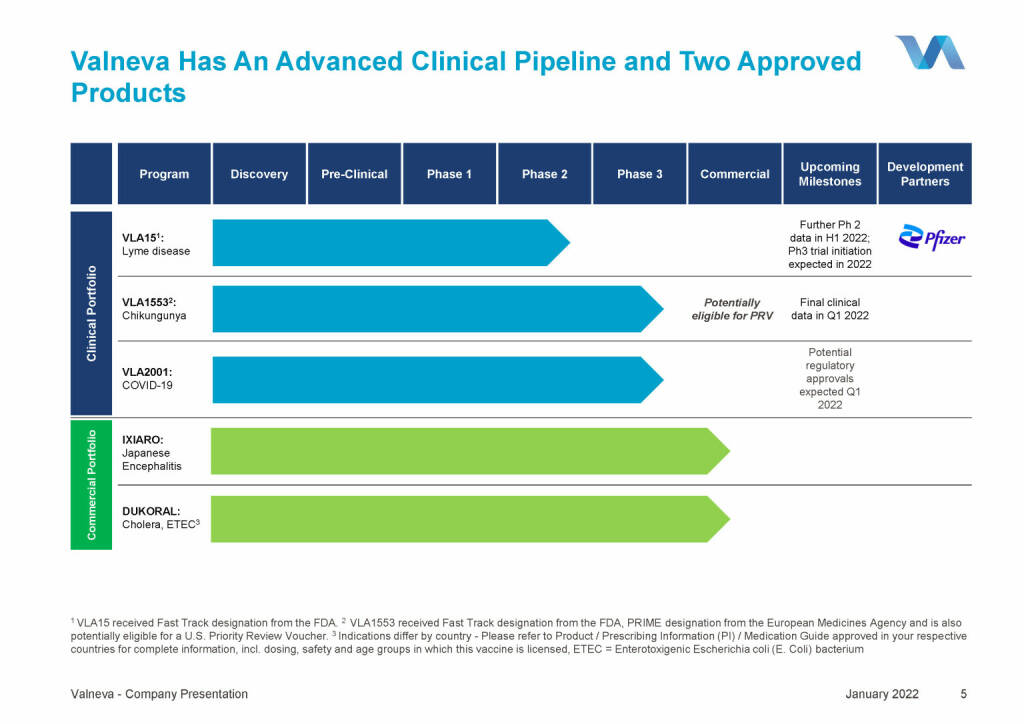
Valneva - Has An Advanced Clinical Pipeline and Two Approved (18.01.2022)

Valneva - Lyme Disease Vaccine – VLA15 (18.01.2022)
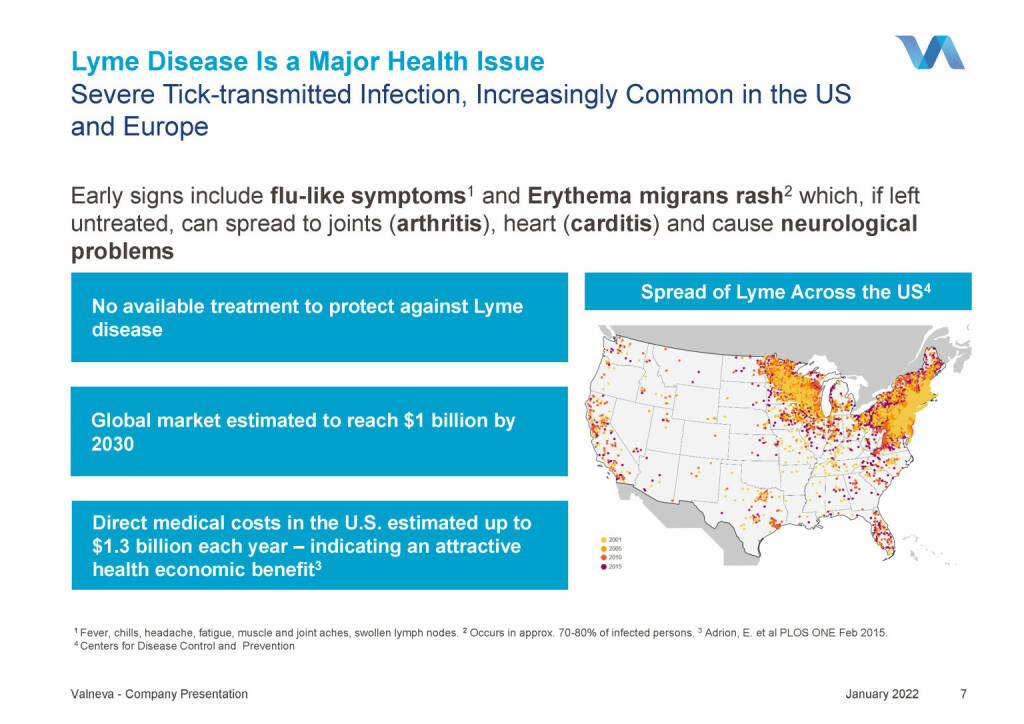
Valneva - Lyme Disease Is a Major Health Issue (18.01.2022)
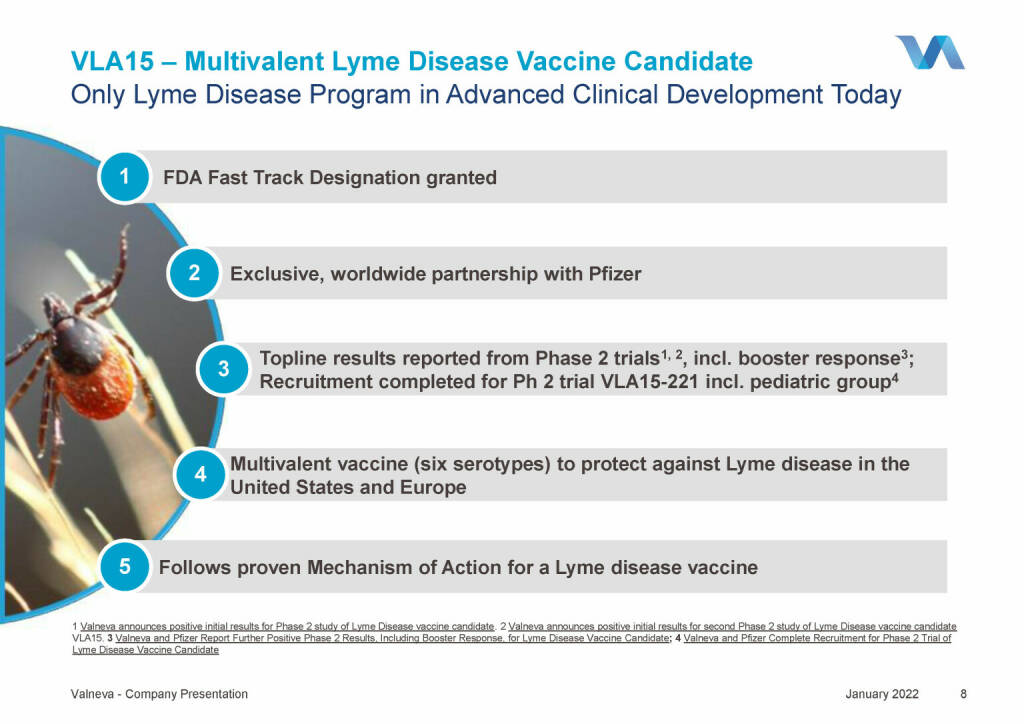
Valneva - VLA15 – Multivalent Lyme Disease Vaccine Candidate (18.01.2022)

Valneva - VLA15: Development Progress and Outlook (18.01.2022)

Valneva - SARS-CoV-2 (COVID-19) Vaccine – VLA2001 (18.01.2022)
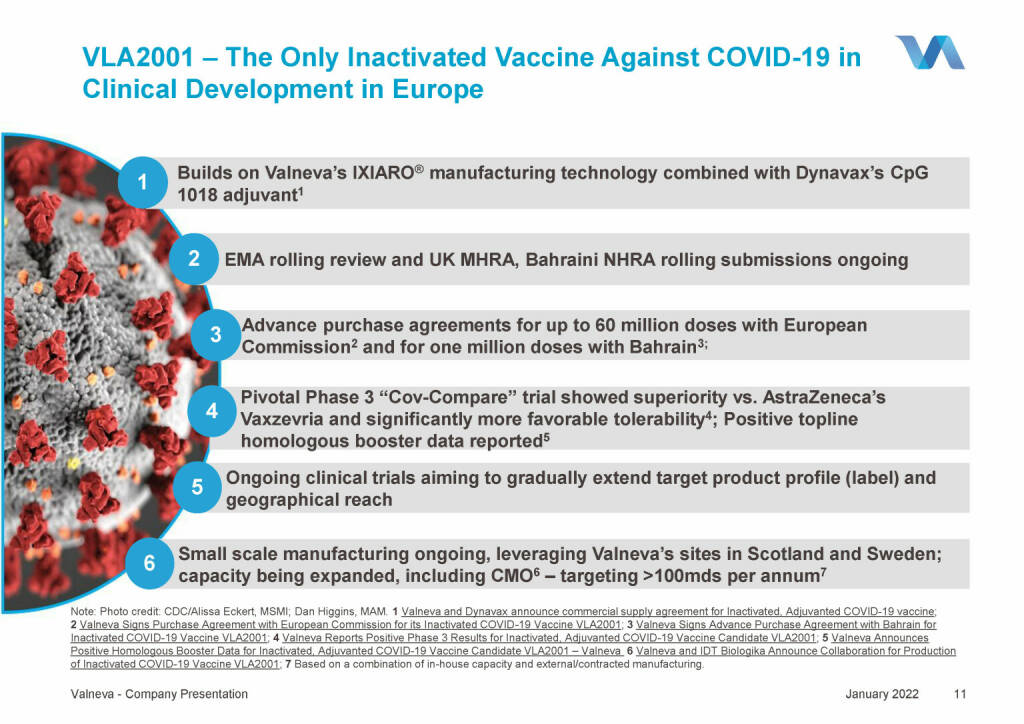
Valneva - VLA2001 – The Only Inactivated Vaccine Against COVID-19 in Clinical Development in Europe (18.01.2022)
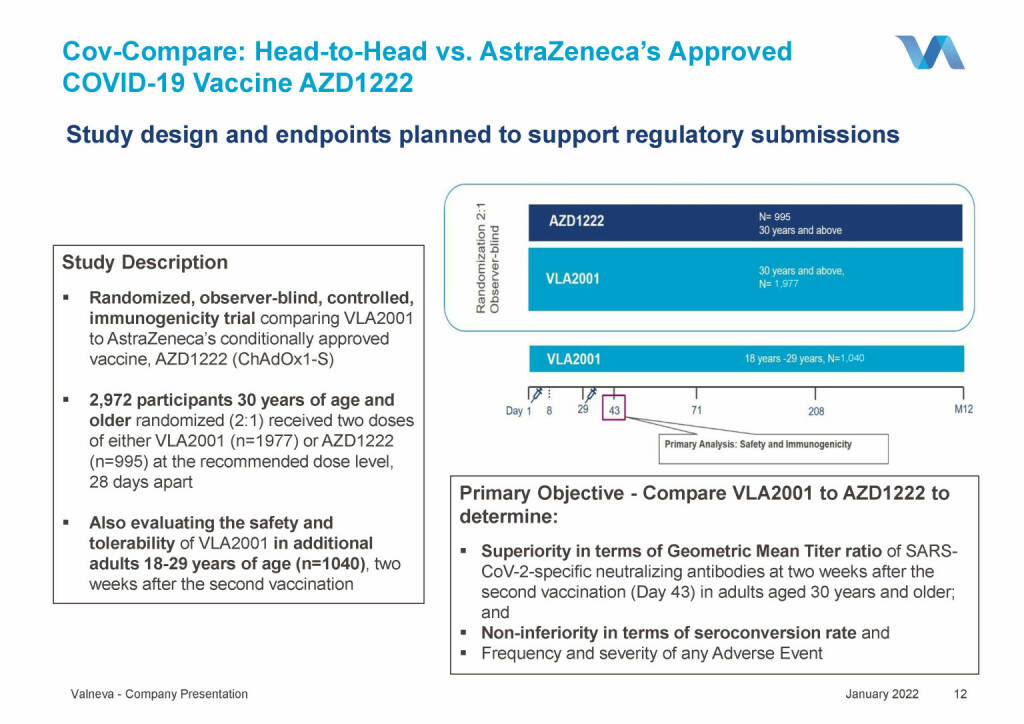
Valneva - Cov-Compare: Head-to-Head vs. AstraZeneca’s Approved COVID-19 Vaccine AZD1222 (18.01.2022)
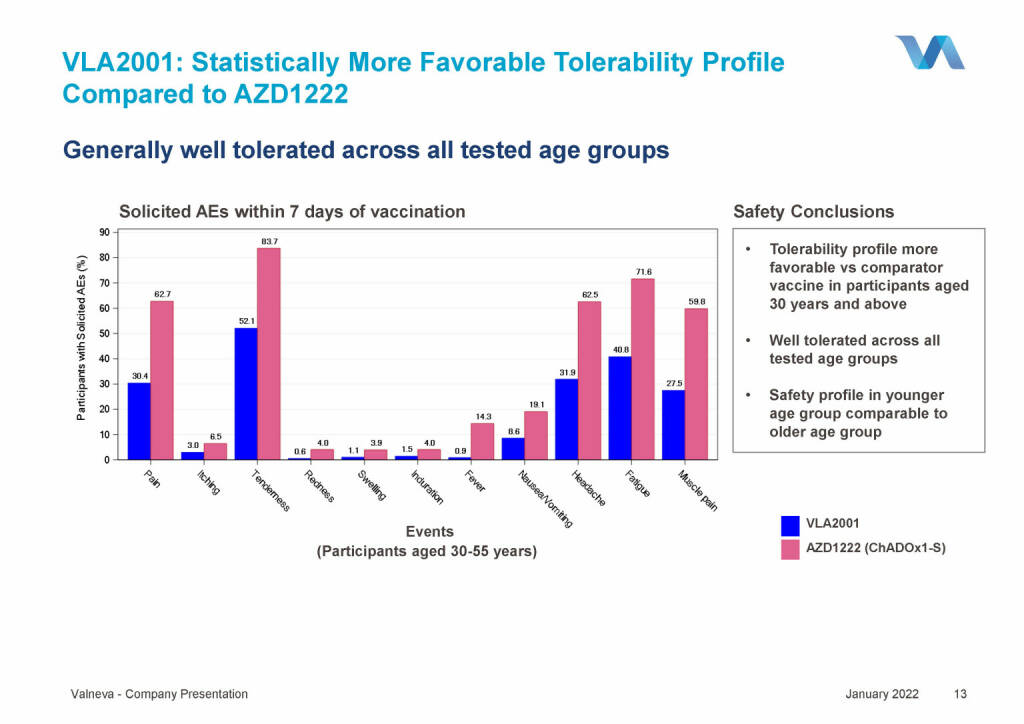
Valneva - VLA2001: Statistically More Favorable Tolerability Profile Compared to AZD1222 (18.01.2022)
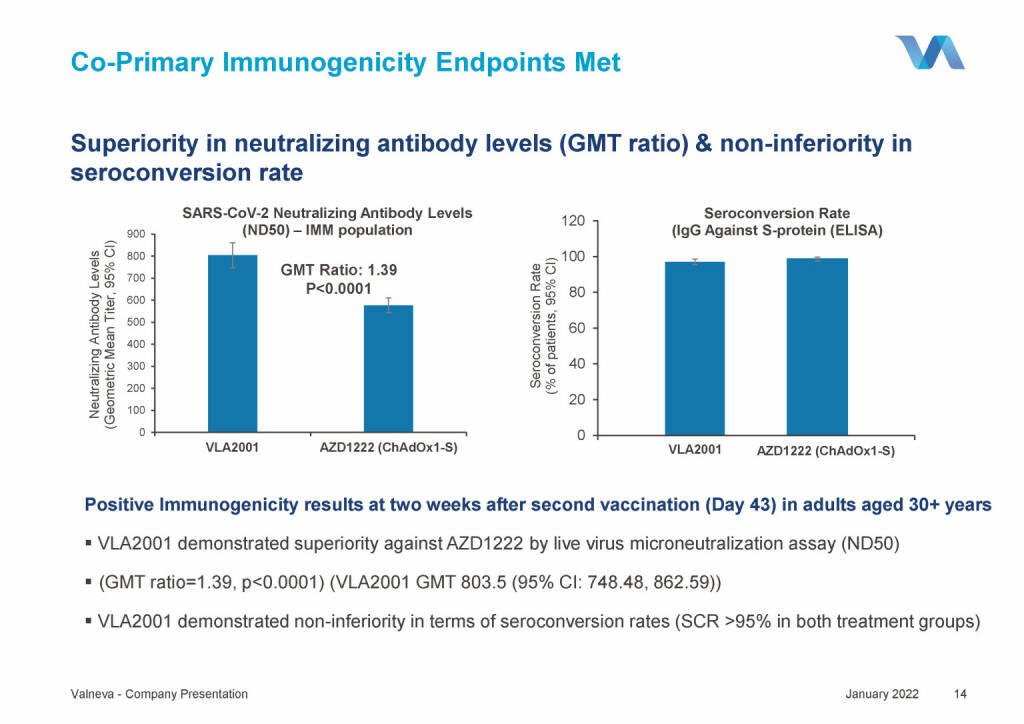
Valneva - Co-Primary Immunogenicity Endpoints Met (18.01.2022)
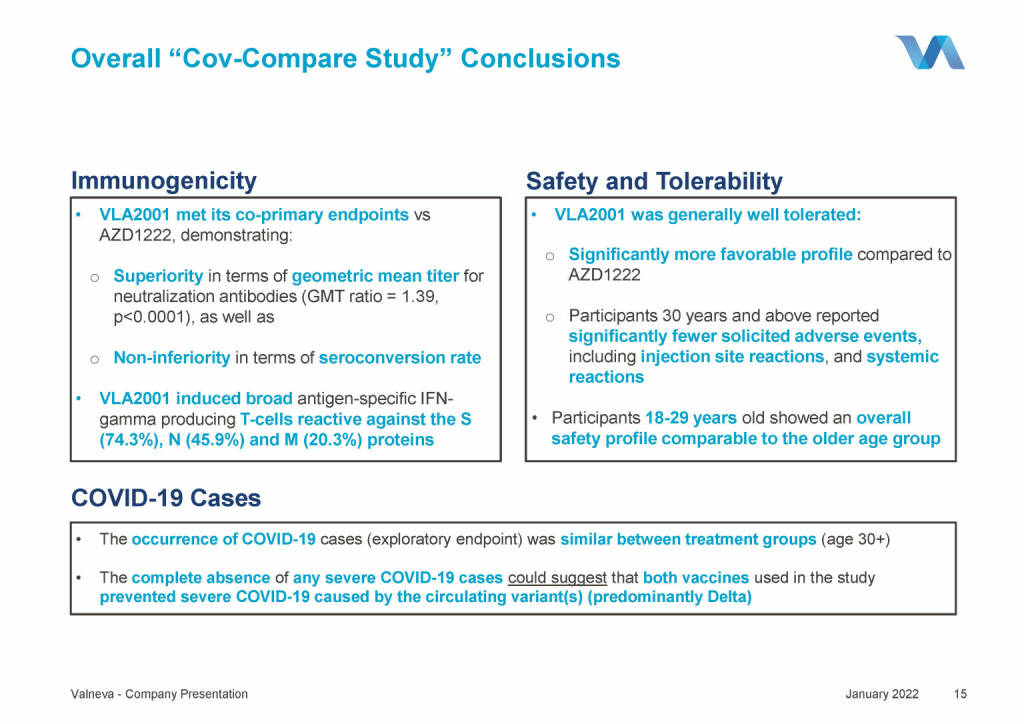
Valneva - Overall “Cov-Compare Study” Conclusions (18.01.2022)
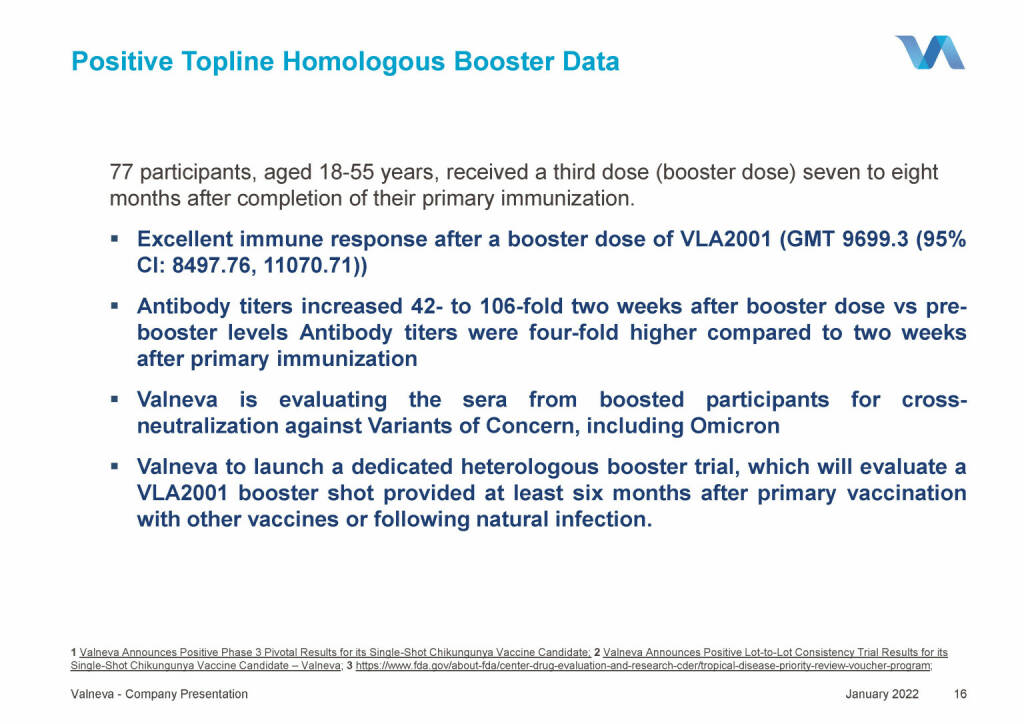
Valneva - Positive Topline Homologous Booster Data (18.01.2022)
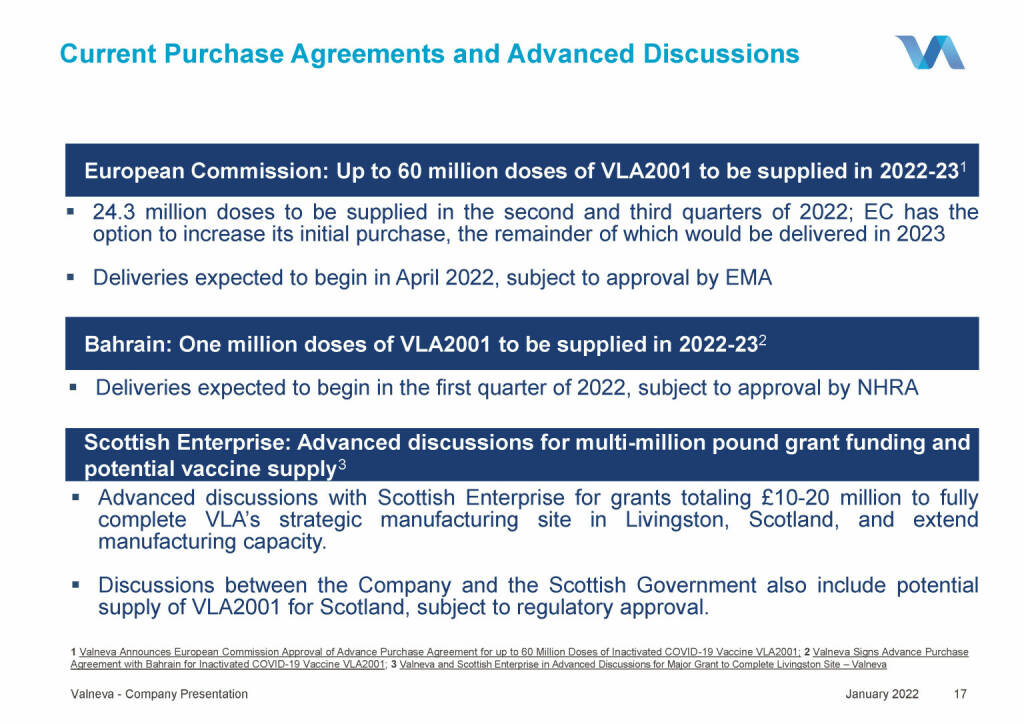
Valneva - Current Purchase Agreements and Advanced Discussions (18.01.2022)

Valneva - VLA2001: Potential to Protect Against Variants (18.01.2022)

Valneva - VLA2001: Value Growth Through Continuous Extension of Label (18.01.2022)

Valneva - Chikungunya Vaccine – VLA1553 (18.01.2022)
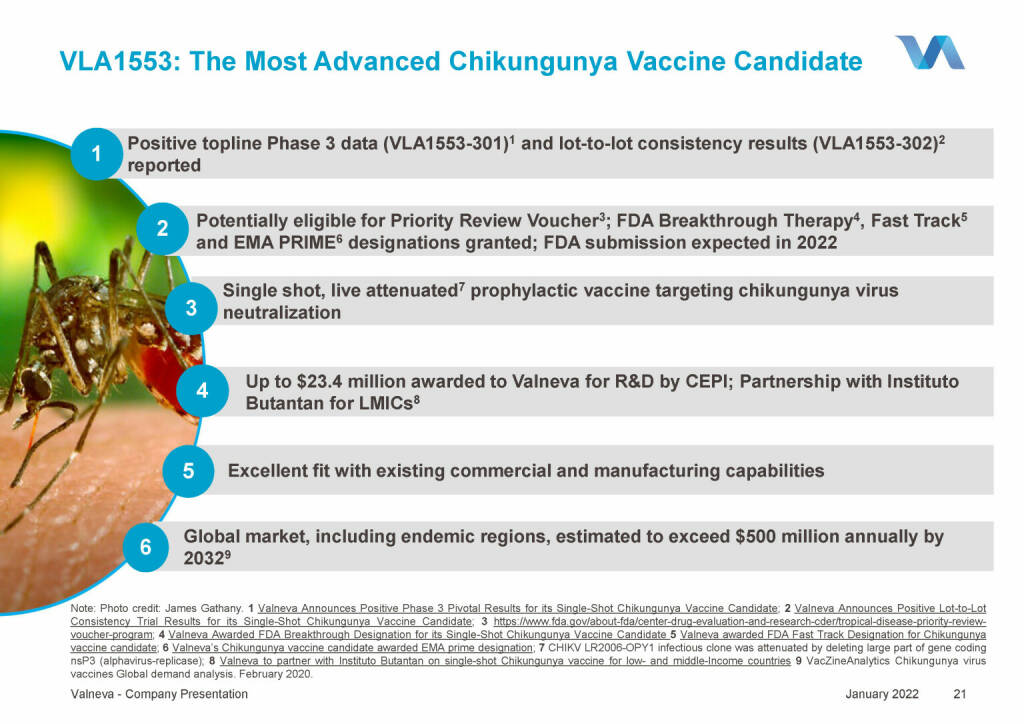
Valneva - VLA1553: The Most Advanced Chikungunya Vaccine Candidate (18.01.2022)
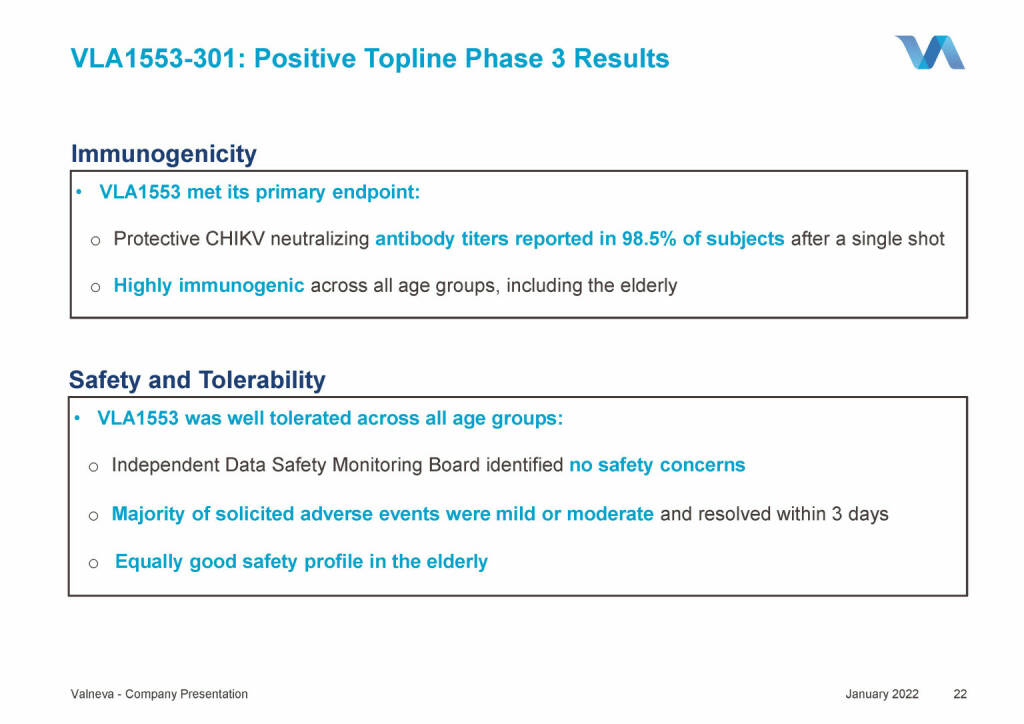
Valneva - VLA1553-301: Positive Topline Phase 3 Results (18.01.2022)

Valneva - VLA1553: Development Outlook (18.01.2022)

Valneva - Commercial Products (18.01.2022)

Valneva - Valneva Has a Specialist Travel Vaccine Business and is a Contractor to the US Military (18.01.2022)

Valneva - Corporate Highlights and Newsflow (18.01.2022)

Valneva - VLA Successfully Raised ~ $210 Million in 2021 (18.01.2022)
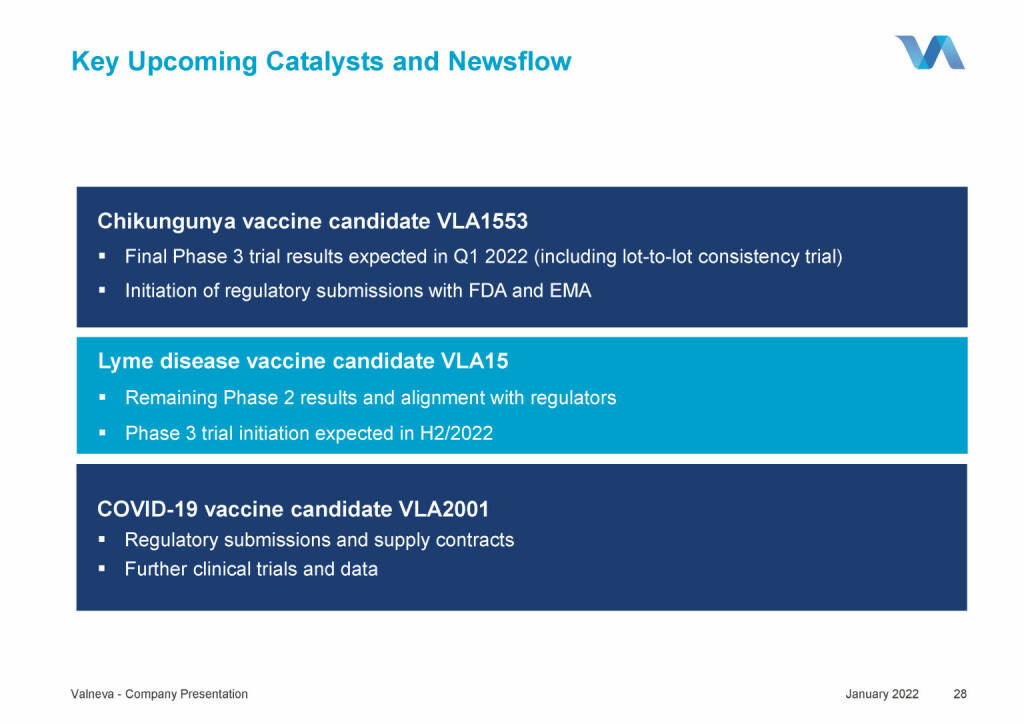
Valneva - Key Upcoming Catalysts and Newsflow (18.01.2022)

Valneva - Thank you (18.01.2022)